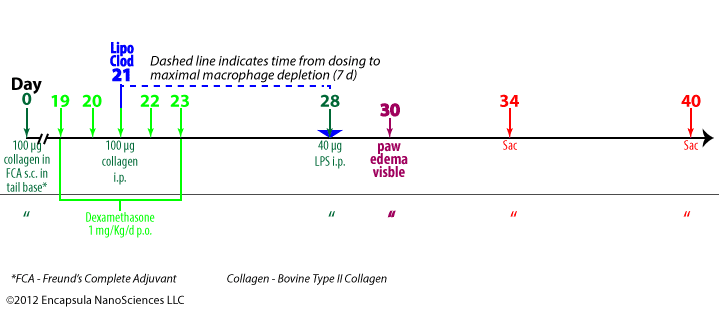
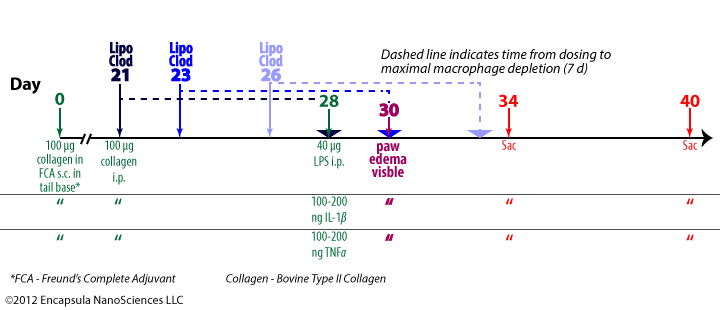
Highton J, Guévremont D, Thomson J, Carlisle B, Tucker I. A trial of clodronate-liposomes as anti-macrophage treatment in a sheep model of arthritis.Clin. Exp. Rheumatol. 1999 Jan;17(1):43–8.
Abstract
Objective
Our previous research has concerned the role of macrophages in joint inflammation in rheumatoid arthritis. We have therefore been interested in liposomes containing clodronate as an anti-macrophage treatment for arthritis. We have used the antigen-induced arthritis model in sheep to evaluate the effect of clodronate liposomes.
Methods
Arthritis was induced in the right hock joint (day 0). We were able to demonstrate uptake of liposomes into macrophages within the inflamed joint lining. On day 7, sheep were given a single intra-articular injection of clodronate liposomes (group 1, n = 10) or saline liposomes (group 2, n = 10). A further 6 sheep (group 3) had no arthritis and no treatment.
Results
No difference in joint diameter was observed between the sheep in group 1 (clodronate) and group 2 (saline treated). Both groups had joint swelling which persisted until the end of the trial (day 20). Histologic scoring was also similar in group 1 and group 2 animals, and both were worse than group 3.
| Clodronate Concentration | Total Lipid Concentration | Lipid Composition | Lipid Mole % | Liposome Type | Control Liposomes | Control Free Clodronate |
|---|---|---|---|---|---|---|
| not specified (see pt. 1) | 42 mg/ml | EPC/BPS/chol | 48/48/4 | MLV | saline | ND |
| Animal Description | Clodronate Dose | Dosing Method/Site | Target Phagocytes | Systemic Dosing? | Systemic Results |
|---|---|---|---|---|---|
| Sheep | 0.5 ml | intra-articular/knee | synovial MΦ | no | N/A |
Notes
- Liposome prep method cited – none.
- We calculated the lipid concentration assuming that no lipid was lost during the preparation of the liposomes; this is a tenuous assumption.
- The author’s stated that the clodronate liposomes were unilamellar, but no size information was provided. While probe sonication can provide small unilamellar liposomes in the range of 50 nm or smaller with this lipid composition, the key is to verify that the liposome suspension converts from an opaque, milky suspension to a translucent suspension which indicates that no large multilamellar liposomes remain in the suspension. The sonication time (5 minutes in this case) required to achieve small unilamellar vesicles (SUV) will vary with volume, vessel geometry, sonicator output, etc., therefore cannot be used to verify that small unilamellar vesicles (SUV) were achieved. The authors did state that the unencapsulated clodronate was removed by centrifugation with no further details. Small unilamellar liposomes are very difficult to pellet by centrifugation but it can be done using an ultracentrifuge and extended centrifugation times of several hours at 100,000xg. If the liposomes are truly SUV, low speed centrifugation (10,000xg range) that is used for multilamellar clodronate liposomes will provide a barely dectable, if present at all, pellet.
- Assuming that small unilamellar liposomes were prepared by the authors as they implied, the problem with SUV is that they encapsulate a very small volume of aqueous phase due to their small internal volume. The aqueous volume of SUV is around 0.5 µl/µmole of lipid or less. The authors reported using 217 µmoles of lipid (by our calculations) which would mean that 108.5 µl of clodronate solution was encapsulated. Again, assuming that the clodronate solution used to rehydrate the dry lipid film was not diluted during the preparation and washing of the liposomes (this is rarely true, and is almost certainly not true in this case since the clodronate solution is hyperosmotic to the saline used for washing the liposomes), then a maximum of 21.7 mg of clodronate would have been encapsulated in the 2 ml clodronate SUV suspension. The authors stated that 200 mg/ml clodronate in liposomes was dosed at 0.5 ml, or 100 mg clodronate, per knee joint; by our calculations, a maximum of 5-5.5 mg of clodronate could have been dosed, if the authors did indeed prepare clodronate SUV-type liposomes. It is very important to characterize the liposome preparations used for treatments at least by assaying the final concentrations of lipid and clodronate in the preparation, yet this is rarely reported in the clodronate liposome literature. Therefore, as in this case, we have no idea of how much clodronate was inside the liposomes that the animals received. We usually make assumptions based on “typical” clodronate liposome preparations, but the liposomes described in this paper are not typical clodronate liposomes.
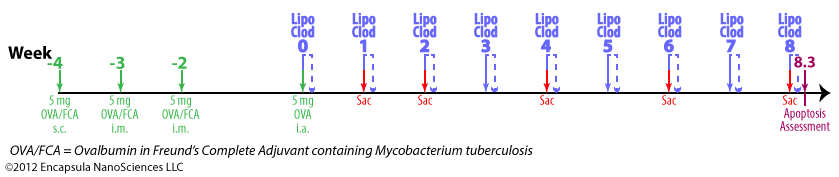
- Antigen-induced arthritis (AIA) was primed by 3 immunization injections of 10 mg ovalbumin (OVA) emulsified in Freund’s Complete Adjuvant (FCA) at 2 week intervals.
- Delayed-type hypersensitivity (DTH) for OVA was assessed 24-48 hr post-antigent injection to ensure a response to OVA.
- AIA was induced by intra-articular injections of 5 mg OVA in 0.5 ml into the right hocks of sheep while the left (control) hock received no injection.
- 7 days later, intra-articular injections of 0.5 ml clodronate liposomes, saline liposomes or steroid(s) were made into the arthritic joints.
- BODIPY-PC-containing liposomes were injected into arthritic hocks to assess the uptake of liposomes into the synovial lining of the joints; no details of liposome prep or dose volume was provided. We assume that the authors prepared them by the same method as used for preparing the control liposomes, but this was not stated in the paper.
Results
- The authors chose the sheep AIA model because the size of the sheep hocks would allow for more accurate intra-articular injections and joint measurements.
- The goal of these experiments was to evaluate the efficacy of liposomal clodronate in the treatment of antigen-induced arthritis (AIA) in sheep, yet the authors did not state the dose of liposomal clodronate that was tested in this model. A 0.5 ml volume of an unknown concentration of liposomal clodronate was dosed intra-articularly to sheep one time at 7 d post-induction of AIA.
- No response was seen with either clodronate or control liposomes with respect to joint swelling.
- The histological analysis seemed to suggest that both clodronate and control liposomes worsened the damage to the joints when compared to untreated arthritic joints.
- When a volume of clodronate liposomes was incubated in heparinized sheep blood ex vivo, the authors reported that liposomes were taken up by monocytes with maximal uptake after 4 h (data not shown).
- When a volume of clodronate liposomes was added to cultured macrophages, the authors reported that macrophages died (data not shown).
- When a volume of clodronate liposomes or control liposomes was added to a monocyte suspension, a respiratory burst was observed and the authors concluded that liposomes caused a pro-inflammatory response.
- When a volume of clodronate liposomes was added to a PMN suspension, similar results were observed leaving us to wonder if the PMN actually endocytosed the clodronate liposomes. Studies in which PMN are incubated with clodronate liposomes usually do not result in the liposomes being taken up by PMN, although there are several reports in the literature of liposome uptake by PMN. However the liposomes prepared in the Highton, et. al. lab may be much smaller than multilamellar clodronate liposomes and contain phosphatidylserine (used only in a few other clodronate liposome studies). Details of the nature of the effect of the clodronate liposomes on PMN which resulted in a respiratory burst would have been a unique contribution to the literature, but…data not shown.
- When a volume of fluorescent liposomes was injected intra-articularly into arthritic sheep hocks, the authors reported that confocal microscopic analysis of the synovial lining at 2, 6 and 24 h indicated that labeled synovial macrophages do take up fluorescent liposomes, however our copy of the paper does not include a colored image for confirmation. The authors further stated that uptake was maximal at 6 h and that “Liposomes had dispersed by 24 hours.”
- Given that this was an atypical clodronate liposome formulation which was not characterized post-preparation, we find little useful data in this report which is unfortunate considering the cost limitations of performing experiments in large animal models.