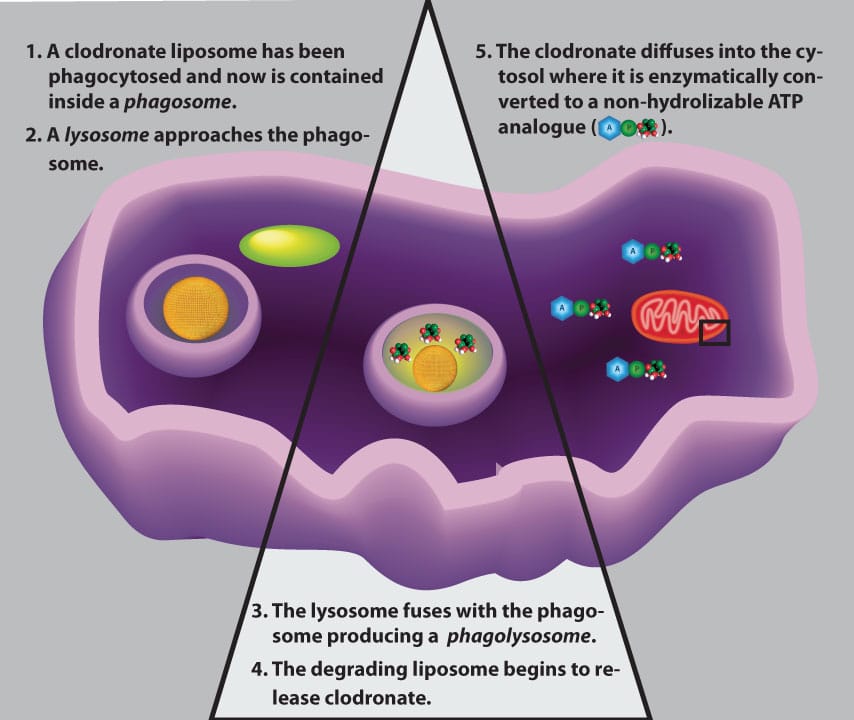
How Do Clodronate Liposomes Induce Apoptosis in Phagocytes?
After Clodrosome has been dosed to the animal by the chosen route, the clodronate liposomes will come into contact with macrophages and other phagocytic cells. The phagocyte immediately recognizes the liposomes as foreign particles and proceeds with destroying these invading particles. The first step in this destruction is phagocytosis in which the liposomes are engulfed by the cell into an internal vesicle known as a phagosome as shown in the figure below. Lysosomes, which contain many types of destructive enzymes, including phospholipases, fuse with the phagosome forming a phagolysosome.
The lysosomal membrane also contains proton pumps which will lower the internal pH of the phagolysosome. The low pH, phospholipases and other macromolecular interactions all contribute to compromising the liposomal membrane thus releasing the encapsulated clodronate. The low internal pH of the phagolysosome may contribute to the ability of the clodronate to cross the phagolysosomal membrane into the macrophage’s cytosol. Once in the cytosolic medium, clodronate is mistakenly recognized as cellular pyrophosphate and used by several Class II aminoacyl-tRNA synthetases to produce a non-hydrolyzable ATP analog, adenosine 5’-( β,γ -dichloromethylene) triphosphate (AppCCl 2 p) [1–3].
[clear]The exact mechanism by which AppCCl 2 p causes cell death remained elusive for some time until Lehenkari and coworkers generated data which supported the hypothesis described in the lower figures. Basically, their hypothesis involves cytosolic AppCCl 2 p crossing the mitochondrial outer membrane and irreversibly binding to the ATP/ADP translocase which transverses the mitochondrial inner membrane. Inhibiting this enzyme initiates pore openings in the mitochondrial inner membrane. Loss of mitochondrial inner membrane integrity, in turn, results in depolarization and allows molecular signals to be released from the mitochondrion which initiate cell death via apoptosis [4].
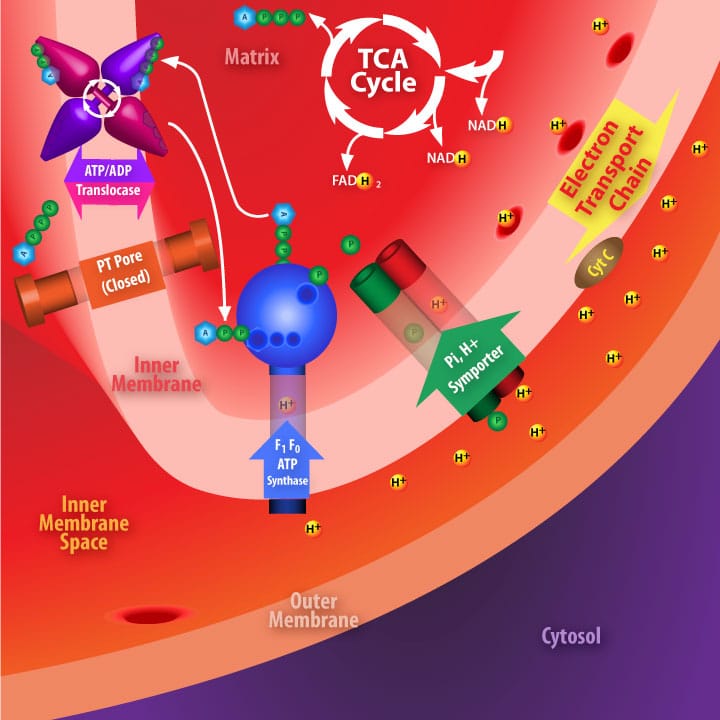
ADP/ATP translocase normally transports one ADP from the cytosol (via the inner membrane space) into the mitochodrial matrix for each ATP transported from the matrix where it diffuses across the inner membrane space to the cytosol.
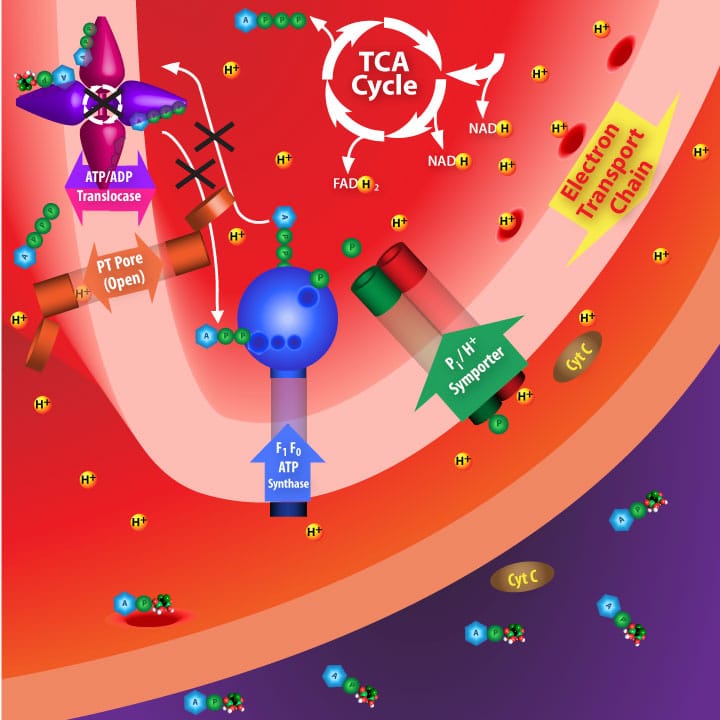
Irreversibly inhibiting ADP/ATP translocase disrupts the electron transport chain due to depolarization across the inner membrane. Some components of the disrupted ETC, such a cytochrome C, migrate to the cytosol and initiate apoptosis.
Thusfar, osteoclasts are the only cells known to internalize free clodronate in vivo, therefore free clodronate is toxic only to these cells in the bone when dosed to animals systemically. The only proven effective method for specifically targeting clodronate to phagocytes in vivo is via liposomes as described above. However, higher concentrations of free clodronate has been shown to kill RAW 264 cells (transformed macrophages) in vitro as well as alveolar macrophages in the lung when the free clodronate is administered directly to the lung [5]. This is presumably due to the inability of free clodronate to escape the specialized endothelium in the lungs allowing a higher concentration of free clodronate to accumulate. If this is the reason for the toxic effects of free clodronate in the lung, perhaps other specialized physiological compartments, such as the cerebrospinal fluid behind the blood-brain barrier, can accumulate toxic levels of clodronate as well. These questions certainly warrant further investigation, but minimally, free clodronate controls must be incorporated into depletion models using non-systemic dosing.
- M.J. Rogers, R.G.G. Russell, G.M. Blackburn, M.P. Williamson, D.J. Watts, Metabolism of halogenated bisphosphonates by the cellular slime mould Dictyostelium discoideum, Biochemical and Biophysical Research Communications, 189 (1992) 414–423.
- M.J. Rogers, D.J. Watts, R.G.G. Russell, X. Ji, X. Xiong, G.M. Blackburn, A.V. Bayless, F.H. Ebetino, Inhibitory effects of bisphosphonates on growth of amoebae of the cellular slime mold Dictyostelium discoideum, Journal of Bone and Mineral Research, 9 (2009) 1029–1039.
- J.C. Frith, J. Mönkkönen, G.M. Blackburn, R.G.G. Russell, M.J. Rogers, Clodronate and Liposome-Encapsulated Clodronate Are Metabolized to a Toxic ATP Analog, Adenosine 5′-( β,γ -Dichloromethylene) Triphosphate, by Mammalian Cells In Vitro, Journal of Bone and Mineral Research, 12 (1997) 1358–1367.
- P.P. Lehenkari, M. Kellinsalmi, J.P. Näpänkangas, K.V. Ylitalo, J. Mönkkönen, M.J. Rogers, A. Azhayev, H.K. Väänänen, I.E. Hassinen, Further insight into mechanism of action of clodronate: inhibition of mitochondrial ADP/ATP translocase by a nonhydrolyzable, adenine-containing metabolite, Molecular Pharmacology, 61 (2002) 1255–1262.
- J.T. Berg, S.T. Lee, T. Thepen, C.Y. Lee, M.F. Tsan, Depletion of alveolar macrophages by liposome-encapsulated dichloromethylene diphosphonate, Journal of Applied Physiology, 74 (1993) 2812–2819.