Čeponis A, Waris E, Mönkkönen J, Laasonen L, Hyttinen M, Solovieva SA, Hanemaaijer R, Bitsch A, Konttinen YT. Effects of low-dose, noncytotoxic, intraarticular liposomal clodronate on development of erosions and proteoglycan loss in established antigen-induced arthritis in rabbits. Arthritis Rheum. 2001 Aug;44(8):1908–16.
Abstract
OBJECTIVE
To assess the clinical and histologic effects of an intraarticular application of low-dose (non-cytotoxic) liposomal clodronate in established antigen-induced monarthritis (AIA) in rabbits.
METHODS
AIA was monitored by assessments of joint swelling, C-reactive protein levels, and radiographic changes in 17 NZW rabbits for 8 weeks during the course of weekly intraarticular injections of liposomal clodronate (0.145 mg/injection, low dose) or “empty” liposomes. The contralateral knee was injected with liposome buffer alone as the control. End-point analyses included macroscopic joint examination, immuno- and TUNEL staining, Safranin O staining/microspectrophotometry, and tumor necrosis factor alpha (TNFα) convertase enzyme (TACE) inhibition assay.
RESULTS
Liposomal clodronate-treated rabbits showed a reduction and delay in joint swelling during the first 3 injections. Expression of matrix-bound (solubilized) TNFα, lining cell hyperplasia, and levels of RAM-11+ macrophages were low in the synovium of the liposomal clodronate treatment group, but the proportion of apoptotic lining cells was not affected. The radiologic score was low at the end of weeks 2 and 4, but at 8 weeks, no difference, compared with controls, was found in pannus formation or in the extent of joint erosion; also, joint swelling was higher than before initiation of treatment. Injections of liposomal clodronate prevented cartilage proteoglycan loss, which was significant in the superficial zone only. TACE activity was not inhibited by clodronate.
CONCLUSION
Liposomal clodronate had temporary antiinflammatory and antierosive effects on established AIA in rabbits. Over the long-term, the loss of cartilage proteoglycans was halted. This observed treatment effect may be related to the inhibition of TNFα production and processing in the synovium.
| Clodronate Concentration | Total Lipid Concentration | Lipid Composition | Lipid Mole % | Liposome Type | Control Liposomes | Control Free Clodronate |
|---|---|---|---|---|---|---|
| 0.29 mg/ml | Not spec. | DSPC/DSPG/chol | Not spec. | REV | saline | ND |
| Animal Description | Clodronate Dose | Dosing Method/Site | Target Phagocytes | Systemic Dosing? | Systemic Results |
|---|---|---|---|---|---|
| New Zealand White rabbits | 0.15 mg/0.50 ml | intra-articular/knee | goal was to avoid depletion of any cell type | no | N/A |
Notes
- Liposome prep method cited – none.
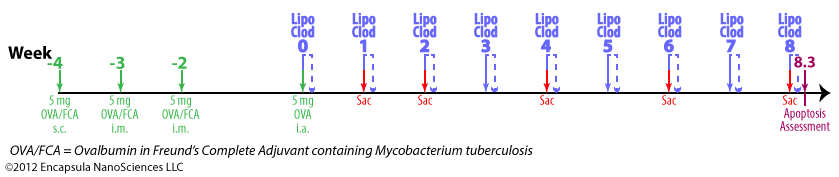
Experimental timeline
- Antigen-induced arthritis (AIA) was primed by 5 mg ovalbumin (OVA) emulsified in Freund’s Complete Adjuvant (FCA) containing 1 mg Mycobacterium tuberculosis .
- The initial inoculation was given subcutaneously at multiple sites in the intrascapular region followed by 2 intermuscular injections given at weeks 1 and 2 (total of 3 inoculations).
- Delayed-type hypersensitivity (DTH) for M. tuberculosis and OVA (intradermal injection) was assessed at week 4 followed by intra-articular injections of 1 ml X 5 mg/ml OVA into one knee while the contralateral knee was injected with 1 ml saline for control.
- Rabbits were given intra-articular injections of clodronate liposomes or control liposomes in the arthritic knees.
Results
- The goal of these experiments was to evaluate non-cytotoxic (no depletion) doses of liposomal clodronate to determine the potential anti-inflammatory effect of clodronate liposomes.
- The authors’ report no synovitis on the first few days after clodronate liposome injection as is seen with higher doses.
- Fluorescent control liposomes injected intra-articularly confirmed the uptake of the liposomes by the synovial lining.
- Arthritis (judged by joint swelling) began within 24 hours of the intra-articular OVA injection and reached significance ( P <0.05 ) by 1 week.
- By week 8 (end of experiment), joints treated with control liposomes were 28% larger than clodronate liposome-treated joints.
- An increase in joint size was delayed until week 6 in the liposomal-clodronate treated animals while the joint sizes began increasing in week 2 for the controls based on the tabular data. However, we are not entirely clear on the authors’ conclusions when compared to the table, therefore we will draw our own conclusions based upon the tabular data.
- There is a significant difference between control and clodronate liposome-treated animals at the end of the experiment when evaluating
- synovial hyperplasia and macrophage density: liposomal clodronate-treated animals showed about 30% less.
- “soluble” TNF in synovium: 66% less in clodronate liposome-treated animals.
- cartilage destruction: lower in clodronate liposome-treated animals.
- No significant differences were observed when evaluating
- cell infiltration into the synovium
- density of CD43+ cells
- expression of TNF by lining cells
- pannus (chronically inflamed synovial tissue) formation
- bone erosion
- The authors’ discuss significant differences between the two groups at various times between 0 and 8 weeks.
- The authors conclude that liposomal clodronate exerts some anti-inflammatory effect beyond that of macrophage depletion (low incidence of apoptotic cells confirmed).
- The authors assume that liposomal clodronate remains active in the synovial fluid for at least one week. We believe that they may have confused the residence time of liposomal clodronate with its ability to maximally deplete synovial macrophages at 7 days. There is no evidence of which we are currently aware that liposomal clodronate, or liposomes, or clodronate remain in the synovial fluid for 7 days. In fact, the authors, themselves, show that this is unlikely because they report the uptake of fluorescent liposomes by synovial cells within the first 6 hours after injection. As we have repeatedly emphasized, liposomes that are phagocytosed will unavoidably be digested by the phagocyte within several hours. These liposomes apparently contained so little clodronate that they were not cytotoxic, as was the goal of the authors, nonetheless, they will be endocytosed within the first 24 hours or so and be subsequently digested. And, until the cell that phagocytoses the liposome dies, the clodronate will remain inside the cell. Clodronate that is released by liposomes before they are endocytosed will quickly diffuse from the synovium. Therefore these animals were exposed to liposomal clodronate for perhaps 24 hours, once per week.
- The authors cited Highton, et. al. (1999) in their claim that liposomes remain in the synovium for 7 days post-intra-articular injection, however we find no such data in this paper. As a matter of fact, Highton, et. al. stated that fluorescent “liposomes had dispersed by 24 hours.” Additionally, a hydrophobic lipid probe (BODIPY-PC) was used in these studies as opposed to the hydrophilic fluorescent probe (calcein) utilized by Čeponis, et. al.
- van Lent, et. al. (1993) do report that “fluorescent liposomes” were still visible in the synovial lining at 7 d, however the presence of the lipophilic fluorescent probe does not mean that intact liposomes were present. As liposomes containing fluorescent lipophilic probes are digested, the probe becomes integrated into the cellular membranes so that the cells and/or intracellular organelles become fluorescent. If there were higher magnification images produced, experienced microscopists might differentiate fluorescent liposomes from intracellular organelles in confocal microscopic images, but these types of images were not shown in this paper. Even if we were to accept that intact liposomes remained in the synovial lining at 7 d, this data cannot be extrapolated to say that intact liposomes containing clodronate would be present. A lipophilic probe can not be used to project the behavior of a hydrophilic compound, such as clodronate. If the goal is to predict the behavior of a hydrophilic compound, then a hydrophilic fluorophore must be encapsulated into the aqueous space of the liposomes as was used by Čeponis, et. al. In this case even if the liposomes retain their basic vesicular structure, but become “leaky” due to opsonization or other enzymatic/macromolecular interactions, the hydrophilic fluorescent probe will be released from the liposomes and the liposomes will loose their punctate fluorescent signal as is very commonly seen in confocal images. The released hydrophilic fluorescent probe will be diluted to a diffuse background fluorescence or some will be quenched by intracellular molecules. However, if punctate fluorescent images persist when liposomes encapsulating hydrophilic fluorophores are introduced into the cells/tissue, the liposomes must be intact.
- In any case, the only way to definitely determine whether clodronate, either still inside liposomes or having been released from the liposomes, is present in either the synovial fluid, lining or sub-lining is to track the clodronate by chemical assay or using radiolabelled clodronate (ideally a double-label study with radiolabelled clodronate encapsulated in radiolabelled liposomes). Currently we know of no evidence that liposomal clodronate would be constantly present in the synovial fluid throughout the experiment as we believe the authors intended and assumed in their data interpretation.